Israel’s Minister of Science and Technology Ofir Akunis congratulated Migal on the “exciting breakthrough” and said he was “confident that there will be further rapid progress, enabling us to provide a needed response to the grave global COVID-19 threat.”
000
Israeli Scientists Work To Test Adapted New Avian Virus Vaccine Against Human Coronavirus
By NoCamels Team March 03, 2020
1
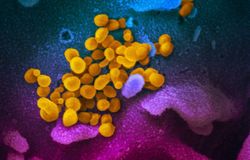
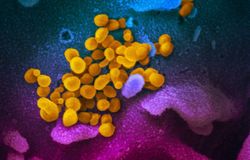
A scientist at the Migal Research Institute's lab where researchers are working on developing a coronavirus vaccine. March 2020. Photo: Lior Jorno
Israeli scientists have announced that a new vaccine they developed for a deadly virus affecting poultry could be adapted for human use against the novel coronavirus known as 2019-nCoV (and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This coronavirus causes the disease called COVID-19 which has claimed the lives of over 3,100 people worldwide, mainly in China, and has been contracted by over 91,000 as of March 3.
Scientists at the Migal Research Institute in the northern Israeli town of Kiryat Shmona said on Thursday that after four years of multi-disciplinary research, they developed a vaccine against IBV (Infectious Bronchitis Virus), an avian coronavirus that affects the respiratory tract, gut, kidney and reproductive systems of domestic fowl. The disease causes “remarkable economic losses to the poultry industry by inducing respiratory and reproductive signs, decreased productive performances and increased mortality,” according to a 2019 veterinary study. The effectiveness of the new vaccine has been proven in pre-clinical veterinary trials, Migal said in the announcement.
000
2
SEE ALSO:Israeli Biomed Firm Says It Developed New Diagnostics Kit For Coronavirus With Results In 25 Minutes
000
Migal is the Israeli Science and Technology Ministry’s regional R&D center in the Galilee region and is owned by the Galilee Development Company Ltd. The institute specializes in biotechnology, computer sciences, precision agriculture, environmental sciences, food, nutrition, and health.
While working on the avian vaccine, the scientists said they identified a possible COVID-19 vaccine candidate as a by-product of the IBV vaccine and have made the “required genetic adjustments to adapt the vaccine to COVID-19, the human strain of coronavirus.” The scientists said they are now working toward safety approvals that will allow for in-vivo testing and – in the future – the production of a vaccine.
Dr. Ehud Shahar, head of the immunology group of the coronavirus research team at Migal, tells NoCamels that the team was actually working on a number of vaccine platforms, one of which was the avian coronavirus, when the novel human coronavirus outbreak began and then spread.
3
The Migal Research Institute’s lab where scientists are working on developing a coronavirus vaccine. March 2020. Photo: Lior Jorno
“It’s a little bit like fate that we were working on this coronavirus vaccine at the same time that the world was suddenly hit by this epidemic of coronavirus for humans,” he says.
Dr. Shahar explains that the avian vaccine will “translate quite easily to a vaccine for humans because the principle is the same – to trigger the immune system to fight it. But one difference is that in human cases, you can take a virus and kill it [this is called an inactivated vaccine, like for the flu or polio] or weaken it [this is a live-attenuated vaccine, like for MMR or smallpox] and create a vaccine that way. And what we created is a synthetic vaccine made of two proteins.”
“It’s also an oral vaccine which has two advantages. One, there’s no need for a shot. And two, the protection is in the mucosal tissues which affect the respiratory and intestinal systems. And we know that this is how COVID-19 works, by affecting these systems,” he says.
Migal said scientific research conducted at the institute has been found that the avian coronavirus has “high genetic similarity to the human COVID-19, and that it uses the same infection mechanism, a fact that increases the likelihood of achieving an effective human vaccine in a very short period of time.”
4
The Migal Research Institute’s coronavirus research team. Courtesy
Dr. Chen Katz, Migal’s biotechnology group leader, noted that “in pre-clinical in-vivo trials, Migal’s researchers have demonstrated that the oral vaccination induces high levels of specific anti-IBV antibodies.”
Migal Research Institute CEO David Zigdon said the center’s goal was now to produce the vaccine over the next two months and achieve safety approval in 90 days, according to the announcement.
000
5
SEE ALSO:Israeli Biomed Firm Says It Developed New Diagnostics Kit For Coronavirus With Results In 25 Minutes
000
“Given the urgent global need for a human coronavirus vaccine, we are doing everything we can to accelerate development…This will be an oral vaccine, making it particularly accessible to the general public. We are currently in intensive discussions with potential partners that can help accelerate the in-human trials phase and expedite the completion [of] final product development and regulatory activities,” added
Dr. Shahar tells NoCamels that the scientists “already have a computational model of COVID-19 to work with and are now expecting DNA virus to get started of the first batch and begin trials.”
“We’ll then start with small animals to see how it works and work our way up to human trials,” he explains, adding that Migal is already in talks with investors and pharmaceutical companies “to start production and to start getting this vaccine out as soon as possible.”
000
6
SEE ALSO:Israeli Robot Assistant Deployed In Asia To Help Minimize Coronavirus Spread
000
“We have four years of research behind us and we are optimistic that we gave a concept for a good vaccine that really works,” he says.
7
Scientists at the Migal Research Institute lab working on developing a coronavirus vaccine. March 2020. Courtesy
Israel’s Minister of Science and Technology Ofir Akunis congratulated Migal on the “exciting breakthrough” and said he was “confident that there will be further rapid progress, enabling us to provide a needed response to the grave global COVID-19 threat.”
Akunis said he gave instructions to the ministry’s director-general to fast-track all approval processes to bring the human vaccine to market “as quickly as possible.”
000
SEE ALSO:Israeli Robot Assistant Deployed In Asia To Help Minimize Coronavirus Spread
000
As health authorities worldwide grapple with the novel coronavirus, a number of private pharmaceutical companies, research organizations and government institutes haverushed to launch efforts to develop treatment.
Last week, US company Moderna announced that it shipped out an experimental vaccine that might protect against the virus. The company’s first batch was sent to the National Institute of Allergy and Infectious Diseases (NIAID) last Monday, The Wall Street Journal reported. US federal scientists will investigate the vaccine and may begin clinical trials as soon as April, according to the report.
California-based biotech firm Gilead Sciences said last week that it has initiated two Phase III clinical studies to evaluate the safety and efficacy of its novel antiviral drug Remdesivir, developed originally for Ebola, in adults diagnosed with COVID-19. These trials build on additional research including two clinical trials in China’s Hubei province – the epicenter of the coronavirus outbreak – led by the China-Japan Friendship Hospital, and a clinical trial in the US-led by NIAID. Results from the studies in China are expected in April, Gilead Sciences said.
China’s National Medical Products Administration approved last month the use of the experimental antiviral drug Favilavir, developed by the Zhejiang Hisun Pharmaceutical Company, in the treatment of the novel coronavirus. Favilavir, developed to treat inflammation of the nose and throat, has demonstrated some efficacy, according to reports, in a trial involving some 70 patients with COVID-19 in the city of Shenzhen.
Chinese authorities are also experimenting with the use of the HIV drug Kaletra/Aluvia (lopinavir/ritonavir) by American biopharmaceutical company AbbVie as part of antiviral treatment. The research is being conducted through coordination with the World Health Organization, the US National Institutes of Health, the Chinese Center for Disease Control, AbbVie said in a statement.
000
Egilsstaðir, 06.03.2020 Jónas Gunnlaugsson
Bloggar | Breytt s.d. kl. 22:45 | Slóð | Facebook | Athugasemdir (1)